药物潜在的致心律失常作用是药物开发过程中一个备受关注的风险因素,当患者伴有低血钾、结构性心脏病、心动过缓等基础疾病时,发生室性快速型心律失常的风险将大大增加。目前已经证明,药物导致心脏复极化的过度延长可能是该风险出现的主要原因[1]。因此在临床前安全性评价研究阶段,通常要进行药物对心室复极化过程的影响评估,其主要参考指标即QT间期。
一、QT间期及评估策略
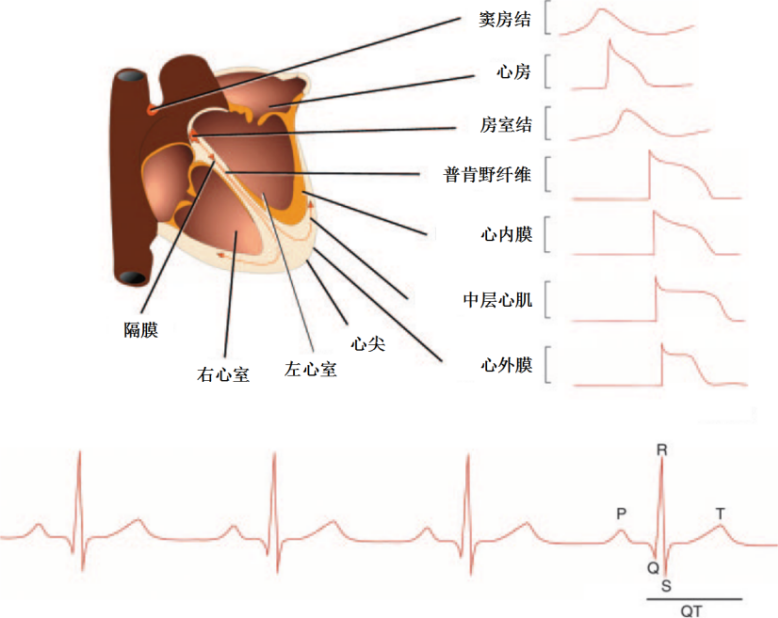
图1 人心肌电生理概图[3]
二、比格犬用于QT间期延长评估的优势 1 ? 应用广泛性

图2 穿戴遥测设备的比格犬
2 ? 离子通道分布的相似性 3 ? 解剖生理学的相似性 4 ? 体内代谢特征的相似性
三、比格犬在安全药理学试验中的应用
四、使用比格犬时QT间期的校正
五、比格犬与人的种属差异
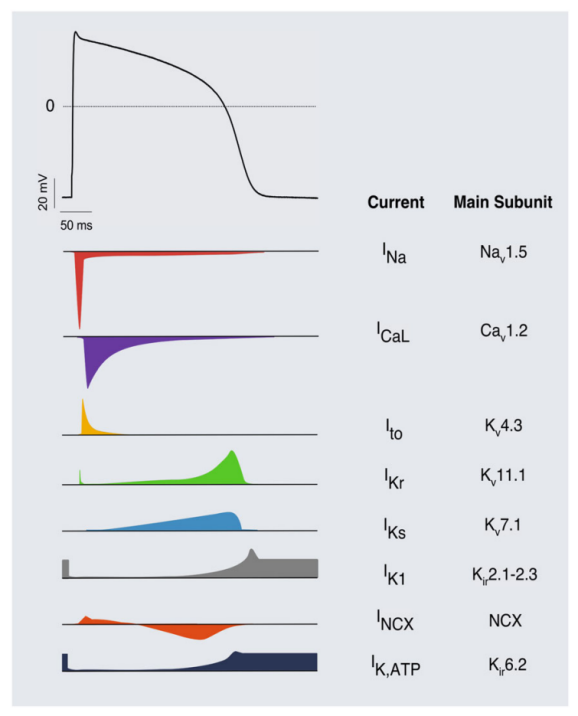
图3 成人心室肌细胞动作电位与离子流图[9]
结语 药物潜在的致心律失常风险是药物开发过程中需要关注的重点。检测动物给药前后QT间期变化是主要的体内评价策略。清醒犬是评估人类药物延长QT间期的主要实验动物。虽然犬与人类在心肌细胞离子通道的分布上存在一定差异,但其对于QT间期延长药物的反应趋势与人类是一致的,结合其代谢动力学特征和经济性,其仍是作为QT间期延长评估的最佳实验动物种属选择。
参考文献:
[1] Dubois VF, Smania G, Yu H, Graf R, Chain AS, Danhof M, Della Pasqua O; Cardiovascular Safety Project Team; TI Pharma PKPD Platform. Translating QT interval prolongation from conscious dogs to humans[J]. Br J Clin Pharmacol. 2017 Feb;83(2):349-362. doi: 10.1111/bcp.13123. Epub 2016 Oct 29. PMID: 27614058; PMCID: PMC5237692.
[2]杨蒙帆,付娜.浅谈QT间期延长[J].中西医结合心血管病电子杂志,2021,9(19):16-18.DOI:10.16282/j.cnki.cn11-9336/r.2021.19.029.
[3] Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization[J]. Physiol Rev. 2005 Oct;85(4):1205-53. doi: 10.1152/physrev.00002.2005. PMID: 16183911.
[4] ICH S7A:Safety Pharmacology Studies For Human Pharmaceuticals[EB/OL].2000.
[5] ICH S7B:The Non-Clinical Evaluation Of The Potential For Delayed Ventricular Repolarization (Qt Interval Prolongation) By Human Pharmaceuticals[EB/OL].2005.
[6] Marostica E, Van Ammel K, Teisman A, Boussery K, Van Bocxlaer J, De Ridder F, Gallacher D, Vermeulen A. Modelling of drug-induced QT-interval prolongation: estimation approaches and translational opportunities. J Pharmacokinet Pharmacodyn. 2015 Dec;42(6):659-79. doi: 10.1007/s10928-015-9434-0. Epub 2015 Aug 11. PMID: 26259721.
[7] Akar FG, Wu RC, Deschenes I, Armoundas AA, Piacentino V 3rd, Houser SR, Tomaselli GF. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am J Physiol Heart Circ Physiol. 2004 Feb;286(2):H602-9. doi: 10.1152/ajpheart.00673.2003. Epub 2003 Oct 2. PMID: 14527940.
[8] Horváth B, Hézs? T, Szentandrássy N, Kistamás K, ?rpádffy-Lovas T, Varga R, Gazdag P, Veress R, Dienes C, Baranyai D, Almássy J, Virág L, Nagy N, Baczkó I, Magyar J, Bányász T, Varró A, Nánási PP. Late sodium current in human, canine and guinea pig ventricular myocardium. J Mol Cell Cardiol. 2020 Feb;139:14-23. doi: 10.1016/j.yjmcc.2019.12.015. Epub 2020 Jan 17. PMID: 31958464.
[9] Jost N, Virág L, Comtois P, Ord?g B, Szuts V, Seprényi G, Bitay M, Kohajda Z, Koncz I, Nagy N, Szél T, Magyar J, Kovács M, Puskás LG, Lengyel C, Wettwer E, Ravens U, Nánási PP, Papp JG, Varró A, Nattel S. Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs[J]. J Physiol. 2013 Sep 1;591(17):4189-206. doi: 10.1113/jphysiol.2013.261198. Epub 2013 Jul 22. PMID: 23878377; PMCID: PMC3779111.
[10] Jost N, Acsai K, Horváth B, Bányász T, Baczkó I, Bitay M, Bogáts G, Nánási PP. Contribution of I Kr and I K1 to ventricular repolarization in canine and human myocytes: is there any influence of action potential duration[J]. Basic Res Cardiol. 2009 Jan;104(1):33-41. doi: 10.1007/s00395-008-0730-3. Epub 2008 Jul 5. PMID: 18604626.
[11] Kalyanasundaram A, Li N, Hansen BJ, Zhao J, Fedorov VV. Canine and human sinoatrial node: differences and similarities in the structure, function, molecular profiles, and arrhythmia[J]. J Vet Cardiol. 2019 Apr;22:2-19. doi: 10.1016/j.jvc.2018.10.004. Epub 2018 Dec 14. PMID: 30559056; PMCID: PMC6436970.
[12] 国家食品药品监督管理局.药物安全药理学研究技术指导原则,2014.
[13]Gralinski MR. The dog's role in the preclinical assessment of QT interval prolongation. Toxicol Pathol. 2003 Jan-Feb;31 Suppl:11-6. doi: 10.1080/01926230390174887. PMID: 12597426.
[14]Tattersall ML, Dymond M, Hammond T, Valentin JP. Correction of QT values to allow for increases in heart rate in conscious Beagle dogs in toxicology assessment. J Pharmacol Toxicol Methods. 2006 Jan-Feb;53(1):11-9. doi: 10.1016/j.vascn.2005.02.005. PMID: 15886026.
[15]李华,邱云良,潘学营等.安全性评价试验中Beagle犬校正QT间期的计算分析[J].毒理学杂志,2007,(04):300.DOI:10.16421/j.cnki.1002-3127.2007.04.123.